Diabetic Macular Edema: Diagnosis and Management
Diabetic retinopathy (DR) is the leading cause of new cases of blindness among adults aged 18 to 64 years in the United States.1 Dia betic macular edema (DME), a severe complication of DR that occurs specifi cally as a result of inadequately treated diabetes mellitus (DM), has overtaken proliferative diabetic retinopathy as the most common cause of vision impair ment in individuals with DM.2 In recent epidemiologic studies, approximately 30% of patients worldwide with DM were found to have visionthreatening DR; and in the United States, 3.8% of patients were found to have DME.3
DME, which is characterized by hard exudates and edema within the macula secondary to damage to retinal microvasculature, is detected by clinical examination or with OCT. Before the advent of pharmacotherapy for DME, the firstline treatment was tradition ally focal laser photocoagulation of the macula. More recently, largescale clinical evidence from the DRCR.net has established intravitreal antiVEGF injections as the firstline therapy, followed by the use of intravitreal corticosteroids if treatment response is unsatisfactory.4
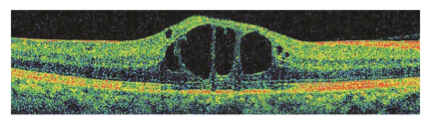
CENTRAL DME. Spectral-domain macular OCT shows central DME with intraretinal cystic spaces and disruption of central retinal architecture.
Etiology and Pathogenesis
DR. DR develops from the loss of both endothelial tight junctions and peri cytes in retinal capillaries, eventually leading to leakage of protein, lipids, inflammatory molecules, and other plasma components into the interstitial space. Further production of proin flammatory cytokines and VEGF by retinal pigment epithelium, glial cells, and macrophages leads to breakdown of the bloodretina barrier, causing further leakage of fluid into the retina.
DME. DME arises from the accu mulation of fluid, protein, and lipids throughout the layers of the retina in the form of intraretinal cystic spaces, best seen by OCT.5 It is now believed that the etiology of DME, though com plex, is largely twofold.
First, retinal microvascular obstruc tion and capillary dropout throughout the retina in patients with poorly con trolled DM lead to retinal ischemia. The subsequent hypoxiainduced upregulation of VEGF then causes neovascularization both in the retinal periphery and in existing macular ves sels, increasing vascular permeability.
Second, in many patients with longstanding DM, production of free radicals and accumulation of advanced glycosylation end products cause upregulation of proinflammatory cytokines such as interleukin (IL)1b and IL6. This process leads to further visionthreatening consequences of DME as inflammation develops and vascular pericytes are lost. Compro mised junctional proteins in macular microcapillaries cause them to become more liable to leakage, contributing to the extravascular fluid and hard lipid exudates that are a hallmark of DME.6
Diagnosis and Screening
Because of the insidious nature of both DR and DME, all diabetic patients should have an ophthalmic evaluation to screen for eye disease, consisting of a comprehensive eye examination, with ancillary testing and imaging as appropriate. According to the Academy’s Preferred Practice Patterns guidelines for DR, patients with type 1 DM should be screened for DR starting five years after diagnosis of DM, while patients with type 2 DM should be screened for DR upon diagnosis and then annually or more often, depending on the severity of their systemic disease.7
Imaging. OCT has become a main stay in screening and diagnosis. This modality allows clinicians to detect thickening, structural changes, and edema that are difficult to capture in a clinical funduscopic exam.
Nonmydriatic or mydriatic digital retinal photography is often used in comprehensive ophthalmology settings for noninvasive screening. It has the potential to be employed in combination with advanced artificial intelligence algorithms that automate the diagnostic process.8,9
Classification. After DME has been detected, the ophthalmologist should perform a detailed clinical examination to determine its severity. DME is typ ically classified in the following three categories:
• Mild: Retinal thickening and hard exudates are present in the posterior pole but fall more than 1,000 μm out side the central macular subfield.
• Moderate: Retinal thickening or hard exudates are present within the central subfield of the macula but do not involve the center.
• Severe: Retinal thickening or hard exudates involve the center of the macula.10
Treatment and Prevention
Treatment of DME begins with management of the systemic disease. Stringent regulation and treatment of hyperglycemia, hypertension, and hyperlipidemia can delay the onset and progression of various microvasculopa thies, including DR and DME.
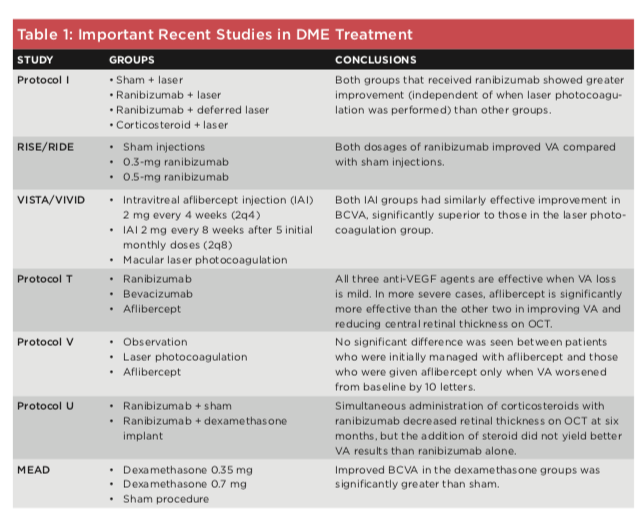
Treatment options for DME vary depending on the severity of disease and the patient’s baseline visual acuity (VA). However, on the basis of recent studies by the DRCR.net, discussed below, ophthalmologists have generally adopted antiVEGF intravitreal therapy as the firstline treatment. (See Table 1 for an overview of important treatment studies.)
Laser. Laser photocoagulation became the primary therapy for DME in the mid1980s, when the Early Treatment Diabetic Retinopathy Study demonstrated its ability to decrease the risk of vision loss. However, the introduction of antiVEGF drugs in the 2000s changed the treatment paradigms because these drugs can reverse vision loss, an outcome that is uncommon with laser therapy.11 The DRCR.net Protocol I study showed a significant improvement in participants treated with ranibizumab and laser therapy (whether on a fixed or flexible schedule) compared with those treated with sham injections and laser therapy.
Anti-VEGF agents. The RISE and RIDE studies, performed in 2010, looked at three groups of patients with a baseline VA of 20/30 or worse: The treatment groups received either 0.3 mg or 0.5mg doses of ranibizumab, and a control group received sham injections. Both treatment groups experienced greater improvement in BCVA than did the control group.12
Similarly, in the VISTA and VIVID studies of patients with central DME, 2 mg of intravitreal aflibercept, admin istered either every four or eight weeks (the latter after five monthly doses), produced visual gains that were far su perior to the results with laser therapy.13
The DRCR.net Protocol T study compared the efficacy of the three anti VEGF drugs currently in widespread clinical use for DME: ranibizumab, aflibercept, and bevacizumab (used off label). Participants were randomly assigned to one of the three treatment groups. The study concluded that aflibercept is the most effective drug in eyes with a baseline VA of 20/50 or worse. There was no significant dif ference in efficacy among the drugs in eyes with better baseline VA.
In the DRCR.net Protocol V study, the investigators compared aflibercept, laser photocoagulation, and observation in the initial management of patients with centerinvolving DME and a base line BCVA of 20/25 or better. No signif icant difference was found, suggesting that in eyes with mild VA loss, the three approaches are equally effective.14
Corticosteroids. In approximately 40% of patients with chronic DME, antiVEGF therapy is unsuccessful or inadequate. Intravitreal corticosteroid therapy is indicated for these patients, as it is presumed that inflammation may be contributing to the pathogen esis of DME. Treatment can be admin istered via intravitreal injection or sustainedrelease intravitreal implants. Physicians considering intravitreal steroids should keep in mind the risks, including premature cataract forma tion, increased IOP, and worsening vision loss.
As a secondline pharmacologic agent for DME, intravitreal corticoste roid implants have been associated with variable outcomes. For example, in the DRCR.net Protocol U study, patients with persistent DME who received intravitreal dexamethasone implants in combination with ranibizumab had decreased retinal thickening on OCT, although BCVA did not improve.
In the MEAD study of a dexameth asone implant, patients who completed the trial had a 0.9 letter gain in BCVA compared with those who dropped out. Among the participants, 37.5% had no change in BCVA, while 23.2% gained more than 10 letters, and 16.0% lost more than 10 letters.15
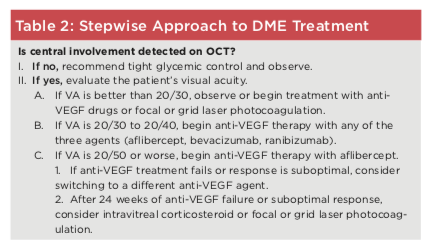
Putting it together. These data suggest a stepwise approach to treat ment (see Table 2), with antiVEGF treatment initiated in patients with moderate to severe DME (VA of 20/30 or worse). Approximately three months or more after starting antiVEGF treat ment, the patient should be reevaluated clinically and with OCT, and further treatment options should be considered if VA and/or central macular thickness have not improved or stabilized sufficiently. If the response to antiVEGF therapy is suboptimal at this point, some retina specialists choose to initi ate intravitreal corticosteroid therapy and focal or grid laser photocoagu lation, while many others prefer to continue with six months of anti VEGF injections before considering intravitreal corticosteroid therapy.
1 CDC. National Diabetes Statistics Report,
2020. www.cdc.gov/diabetes/pdfs/data/statistics/ nationaldia betesstatisticsreport.pdf. Accessed Dec. 27, 2020.
2 Yau JW et al. Diabetes Care. 2012;35(3):556564. 3 Varma R et al. JAMA Ophthalmol. 2014;132(11): 13341340.
4 Maturi RK et al. JAMA Ophthalmol. 2018;136(1): 2938.
5 Joussen AM et al. Am J Pathol. 2001;158(1):147 152.
6 Wong TY et al. Nat Rev Dis Primers. 2016;2: 16012. doi:10.1038/nrdp.2016.12.
7 American Academy of Ophthalmology, Retina/ Vitreous Panel. Diabetic Retinopathy Preferred Practice Pattern Guidelines. 2019. aao.org/pre ferredpracticepattern/diabeticretinopathyppp. Accessed Feb. 22, 2021.
8 Mitchell P, Wong TY; Diabetic Macular Edema Treatment Guideline Working Group. Am J Oph- thalmol. 2014;157(3):505513.
9 Fenner BJ et al. Ophthalmol Ther. 2018;7(2): 333346.
10 Cheung CY et al. Asia-Pac J Ophthalmol. Published online April 24, 2019. doi:10.22608/ APO.201976.
11 Cantrill HL. Int Ophthalmol Clin. 1984;24(4): 1329.
12 Bressler NM et al. Ophthalmology. 2014;121 (12):24612472.
13 Korobelnik JF et al. Ophthalmology. 2014; 121(11):22472254.
14 Baker CW et al. JAMA. 2019;321(19):18801894. 15 Boyer DS et al; Ozurdex MEAD Study Group. Ophthalmology. 2014;121(10):19041919.
Ms. Elyasi is a medical student at the Bruce and Ruth Rappaport Faculty of Medicine at the Tech nion Israel Institute of Technology, Bat Galim, Haifa, Israel.
Dr. Hemmati is a cornea specialist and chief medical officer of Optigo Biotherapeutics, Vancouver, B.C., Canada.
Relevant financial disclosures: Ms. Elyasi: None. Dr. Hemmati: Cell Care Therapeutics: C,O; Optigo Biotherapeutics: E,O.
See disclosure key, page 10. For full disclosures, see this article at aao.org/eyenet.



